Background:
Asciminib (ASC) monotherapy has been approved for chronic myeloid leukemia (CML) treatment in the patients who failed at least 2 lines of tyrosine kinase inhibitor (TKI) therapy. Theoretically, dual inhibition of the ABL1 kinase protein is plausible by combining ASC with ATP-binding pocket inhibitors (ABPIs) including Imatinib (IMA), Dasatinib (DAS), Nilotinib (NIL), Bosutinib (BOS) or Ponatinib (PON). A phase 1 study determined the appropriate dose of ASC with fixed doses of ABPIs (IMA, DAS, NIL). However, this approach of using full dose ABPIs with variable dose of ASC would raise a concern of tolerability given that all the toxicity from the doses of ABPI will be augmented by adding even half dose of ASC, evidenced by the FASCINATION trial (Ernst et al. EHA 2023).
Questions still remained such as what is the best dose for combination? Is the combination of full dose ASC with reduced dose ABPIs effective? Is this combination effective to other ABL1 kinase domain mutant CML? For the ABL1 kinase domain (KD) mutant CML, which ABPI and what dose is more effective and more synergistic than other ABPIs?
Methods and Materials:
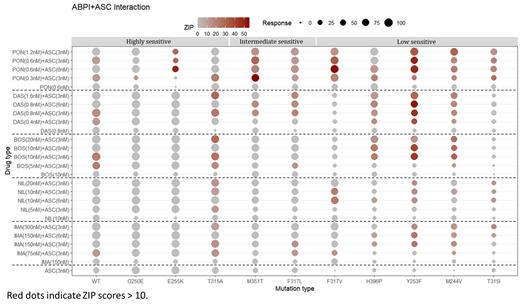
To investigate the combined effect of ASC with ABPIs, we determined the IC 50 values for ASC and ABPIs in the wild-type (WT) BaF3 BCR-ABL+ cell line, and utilized the IC 50 as baselines throughout the study. From the baseline IC 50s of ASC (3nM), IMA (150nM), NIL (10nM), BOS (10nM), DAS (0.8nM) and PON (0.6nM), serially diluted ASC alone, ABPIs alone or in combination were administered to BaF3 cell lines. We conducted IC 50 determining experiment in different combination of ASCs/ABPIs/combination in different concentration (conc) on eleven different KD mutant (KDM) BaF3 cell lines, including WT, G250E, E255K, T315A, M351T, F317L, F317V, H396P, Y253F, M244V, and T315I mutant cell lines. ZIP synergy scores for these cell lines were analyzed using SynergyFinder.
Results
We observed that the addition of 3 nM ASC to ABPI at baseline IC 50 conc led to a higher response (% cell growth inhibition) compared to treatment with ABPI alone at baseline conc, irrespective of the mutation. Moreover, the combined treatment of ABPI at half baseline conc with 3nM ASC demonstrated similar or even higher effects than ABPI alone at IC 50 baseline conc. These results suggest that double blockade allows for the reduction of ABPI dose by half while maintaining cell inhibition activity.
According to its sensitivity to ASC monotherapy and combination, we have stratified the cell lines into 3 categories.
High sensitive cell lines to ASC monotherapy (i.e. WT, G250E, and E255K) achieving 100% of response rate when combined with ABPI. Also, T315A mutation showed a significant synergistic effect (ZIP = 12.91~21.75) with the combination of ASC 3nM with a half conc of ABPI, resulting in 1.90-2.43 times higher inhibitory effect than ABPI monotherapy.
Intermediate sensitive cell lines (i.e. M351T and F317L) displaying 69-85% inhibition efficiency with ASC 6nM combined with ABPI, without significant increases in all ABPI conc.
Low sensitive cell lines (i.e. F317V, H396P, Y253F, M244V, and T315I) exhibiting unique resistance profiles that varied with different combinations. Notably, F317V and F317L mutations showed resistance to DAS. When combined with ASC 3nM, F317V showed restored sensitivity with IMA or NIL than with DAS, while F317L better with DAS. Moreover, the H396P, Y253F, and M244V mutations demonstrated higher sensitivities with BOS or DAS. Interestingly, in the combination treatment with ASC at 3 or 6 nM, with exception of the F317V and T315I mutations, both DAS and PON exhibited a potent efficacy. High resistance of T315I to ABPIs can be overcome with combination of ASC at baseline conc with reduced dose PON. In the case of T315I, the combination of 3nM ASC with a half conc PON resulted in a higher inhibitory effect than baseline conc PON alone.
Conclusion:
The present study demonstrated that a half of baseline conc ABPIs in combination with ASC is as effective as other ABPIs not just in WT but also in other ABL1 KDM CML cell lines. Different CML cell lines harboring different ABL KDM has different treatment spectrum on optimal ABPI drug as well as optimal drug conc. This result will be useful to design future clinical trial of dual blockade of ASC with other ABPIs considering the ABL KDM profiles. It also emphasizes the importance of ABL KDM testing prior to ASC monotherapy or combination with other ABPIs.
Disclosures
Kimura:OHARA Pharmaceutical Co., Ltd.: Honoraria, Patents & Royalties, Research Funding. Kim:Novartis: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Honoraria, Research Funding; Merk: Consultancy; Sanofi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal